The Functional Groups
Here, I’m using humour and character-based visuals as revision aids, each functional group has its own slightly unhinged cartoon “resident” designed to make the chemistry stick. The science is kept accurate, but the explanations are light-hearted on purpose to help you recall them under exam pressure.
Click on each molecule to open its page and you’ll find clearer structures, proper notes, and how that functional group shows up in herbal medicine.
Hydrocarbons
Hydrocarbons are the simplest organic molecules, just carbon and hydrogen, no oxygen or nitrogen. They form the basic skeletons that more complex functional groups are built onto.
In herbal and phytochemistry, many key constituents, especially terpenes and essential oil components, start as hydrocarbon frameworks, then gain extra groups (like –OH, –CHO, –COOH) to fine-tune their behaviour.
On this page we’re looking at the main hydrocarbon families:
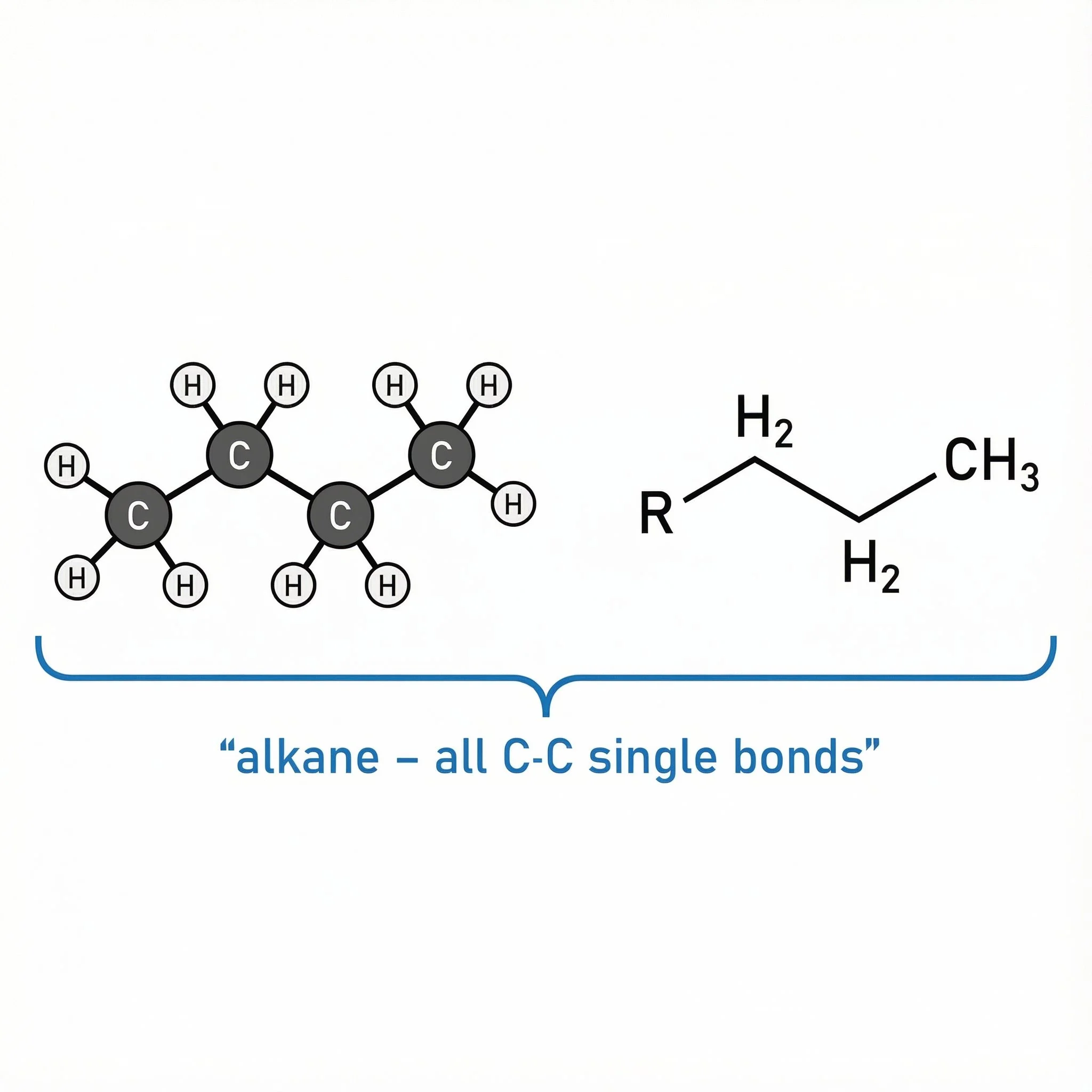
Alkanes – saturated, only C–C single bonds
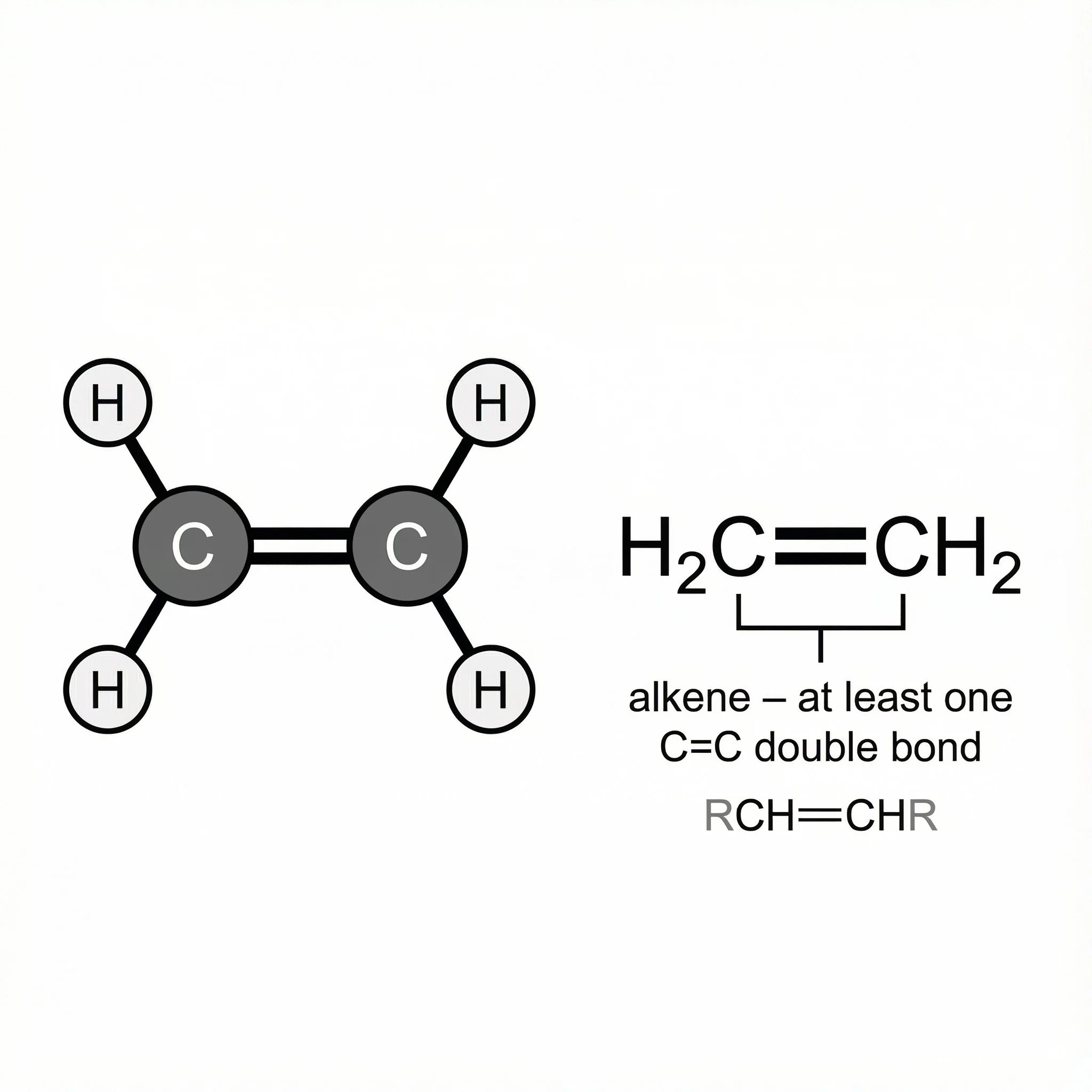
Alkenes – at least one C=C double bond
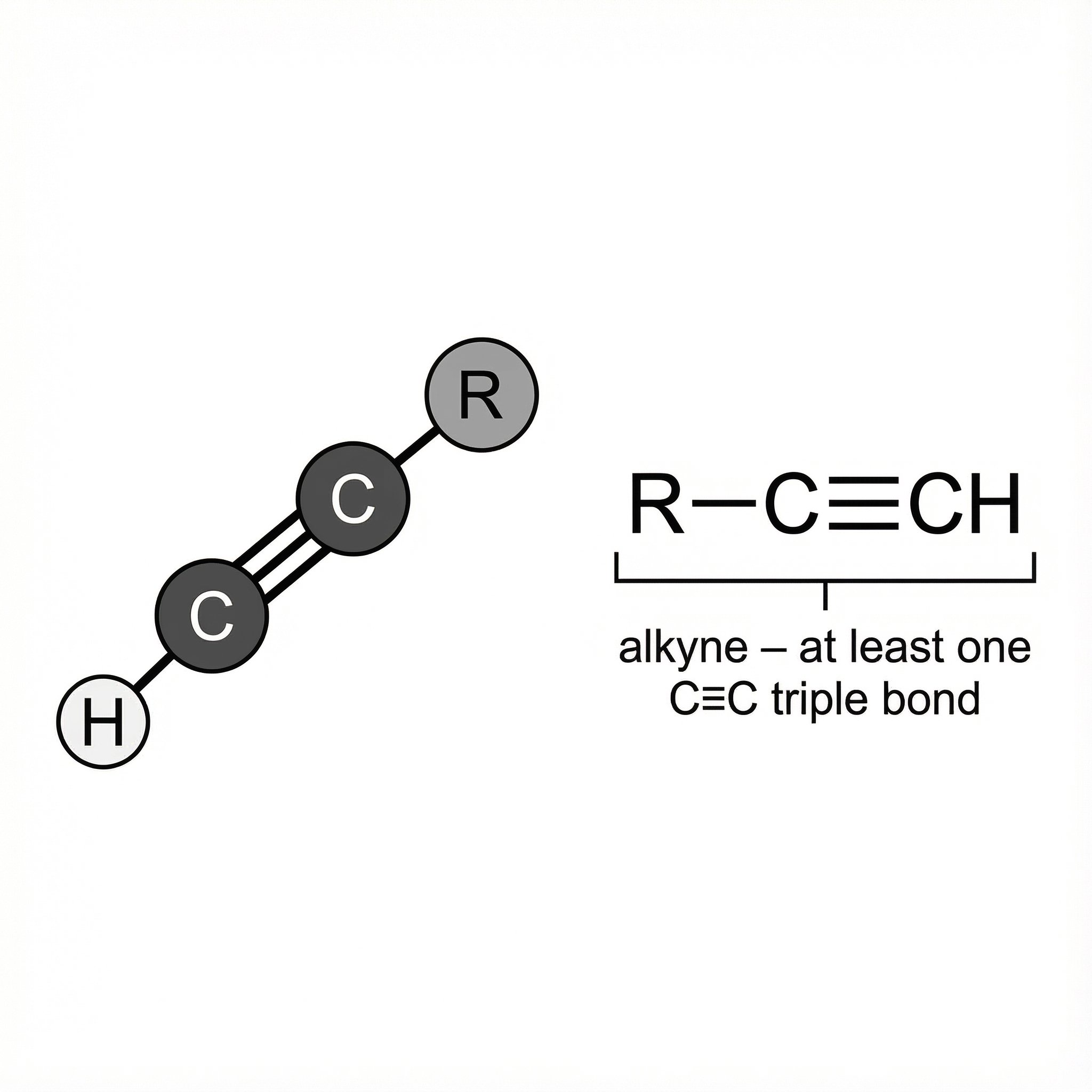
Alkynes – at least one C≡C triple bond
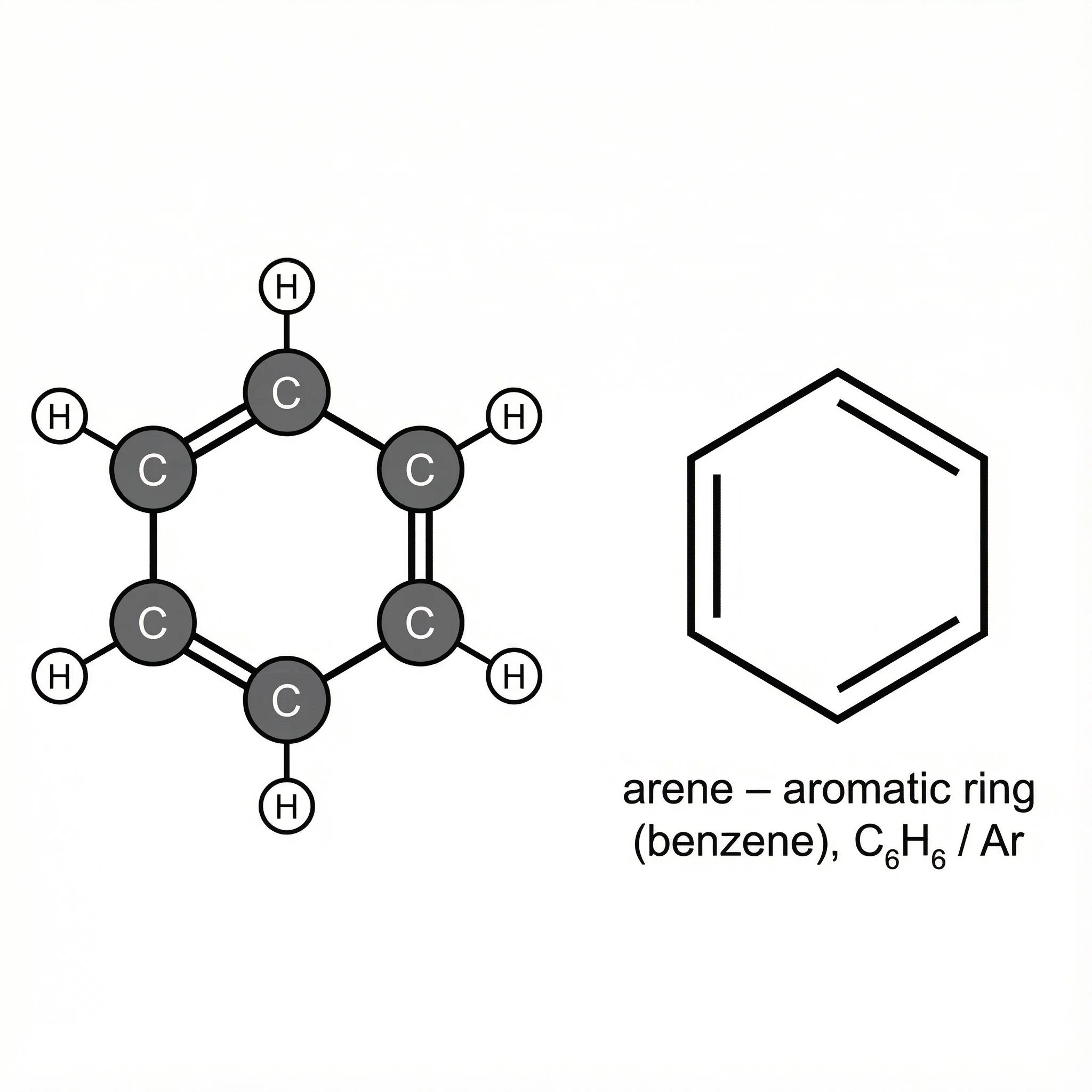
Arenes – aromatic rings such as benzene, forming the core of many aromatic plant compounds.
Simple Oxygen Heteroatomic
Once oxygen turns up, carbon chains stop quietly minding their own business and start interacting with everything. Here we’re looking at the simple, pre-carbonyl oxygen groups, no C=O yet, just oxygen changing polarity, solubility and boiling point.
Click on the molecules to look at simple oxygen heteroatomic compounds of:
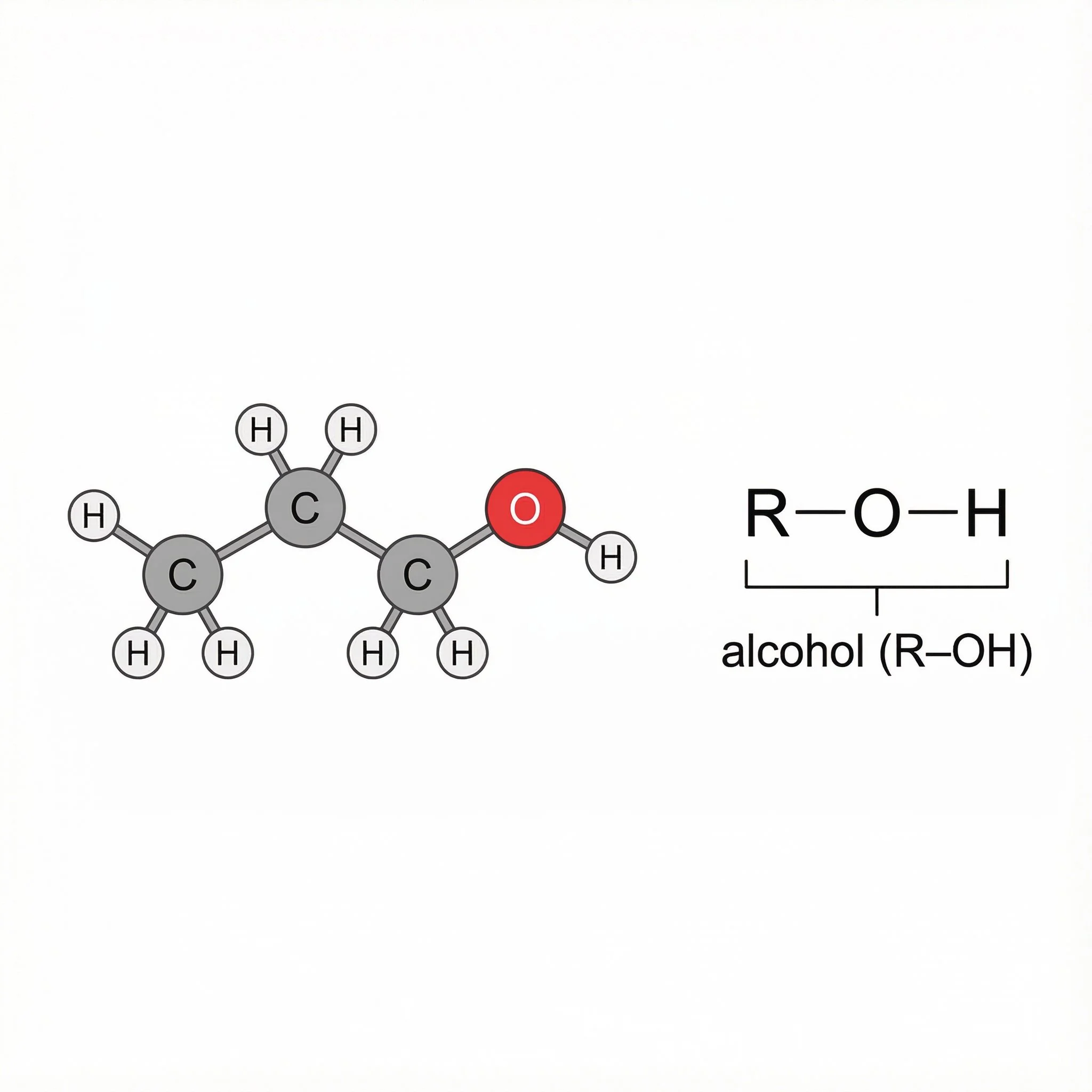
Alcohols (–OH) - at The O–H Arms, where an O–H group hangs off the carbon chain and loves hydrogen bonding.
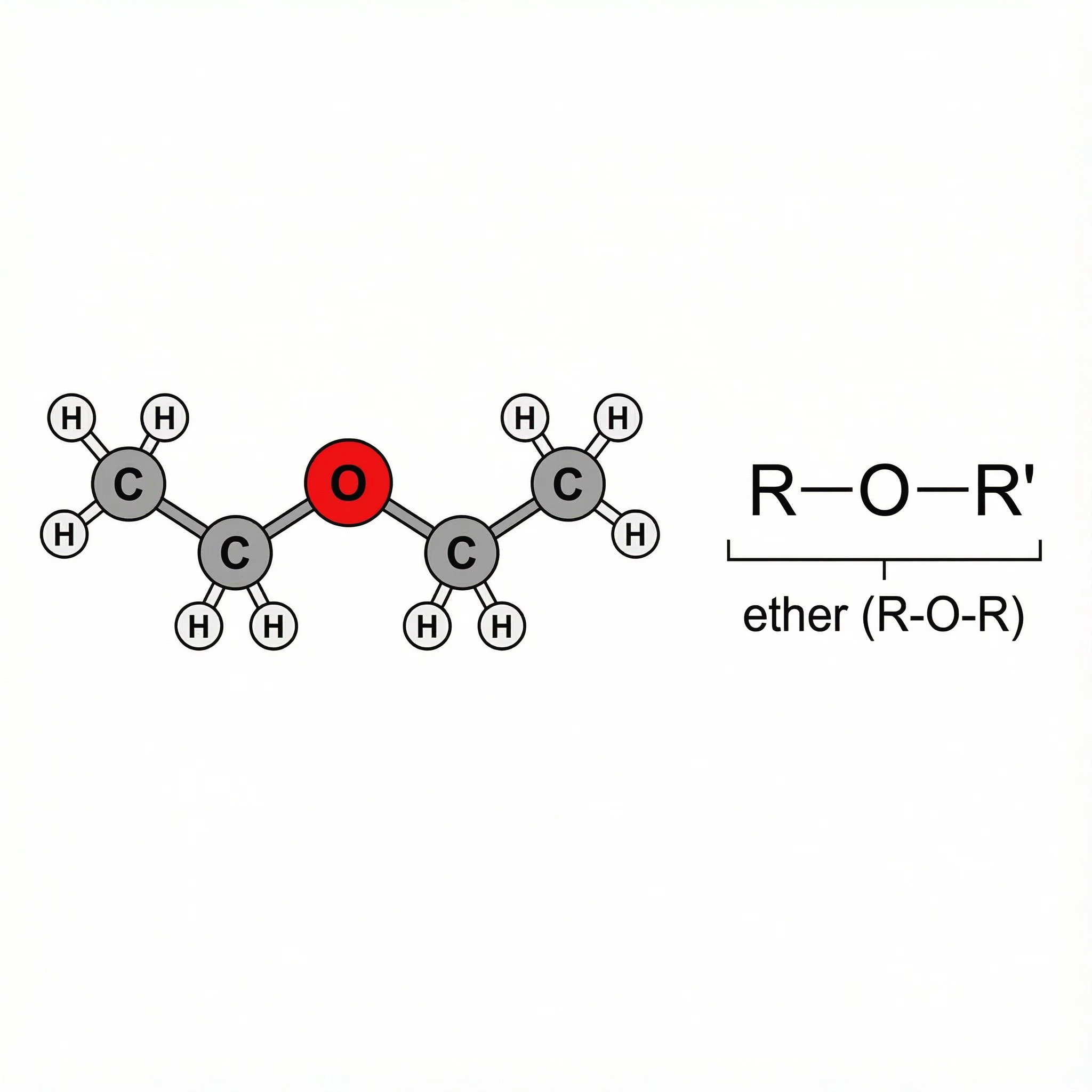
Ethers (R–O–R) - at Club Ether, where oxygen sits between two carbons as C–O–C, behaving more calmly but still subtly influencing how a molecule behaves in plant chemistry.
Carbonyl Compounds
Add a carbonyl group (C=O) and the whole molecule changes personality. Electrons shift, polarity increases, and suddenly it can take part in a much wider range of reactions, oxidation, reduction, nucleophilic attack, acid–base, you name it. Carbonyls are one of the main ways oxygen makes organic molecules chemically “interesting”.
Click on the molecules to look at the main carbonyl-based families you’ll keep meeting in both organic chemistry and herbal constituents:
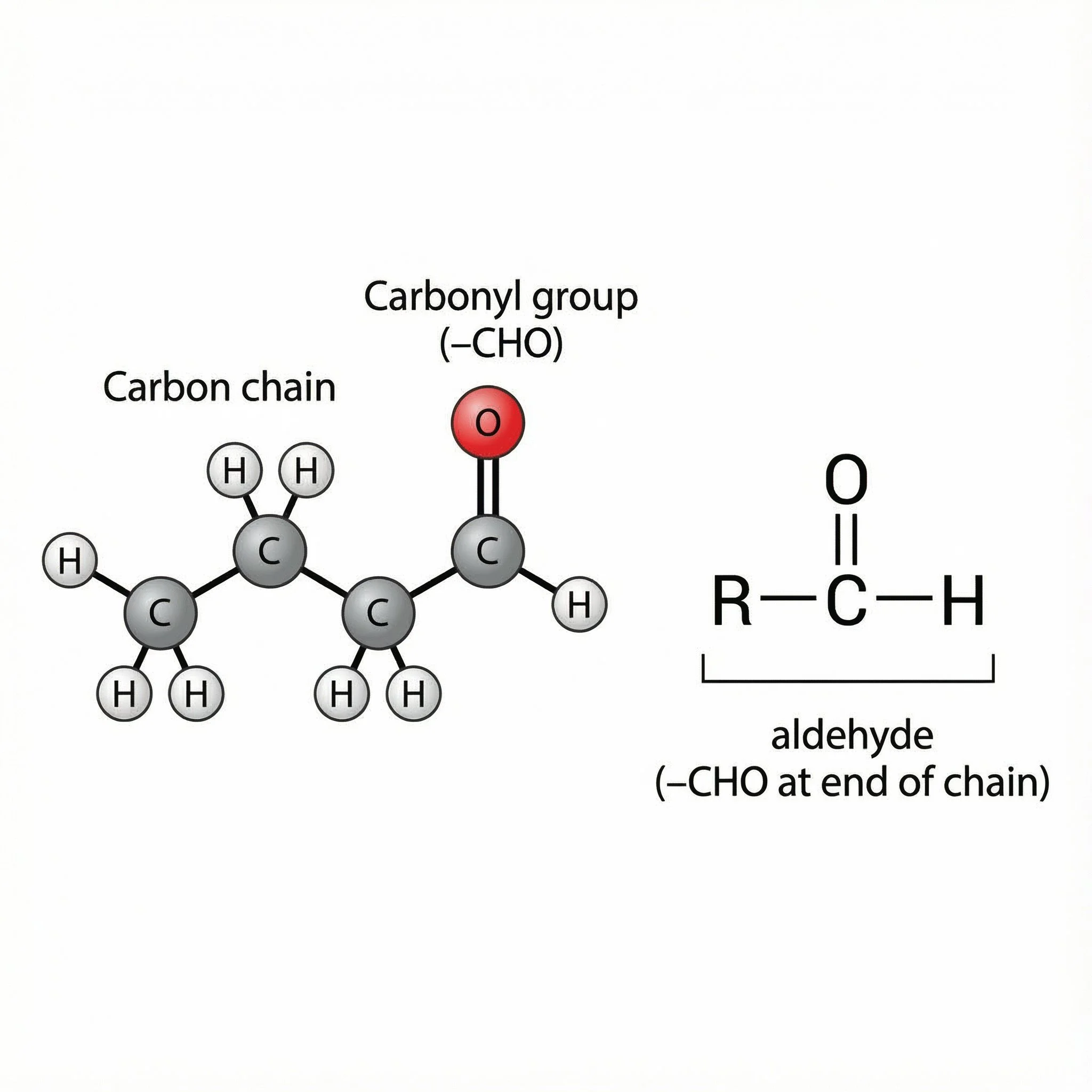
Aldehydes (–CHO) – carbonyls at the end of a chain, quite reactive and often strongly scented.
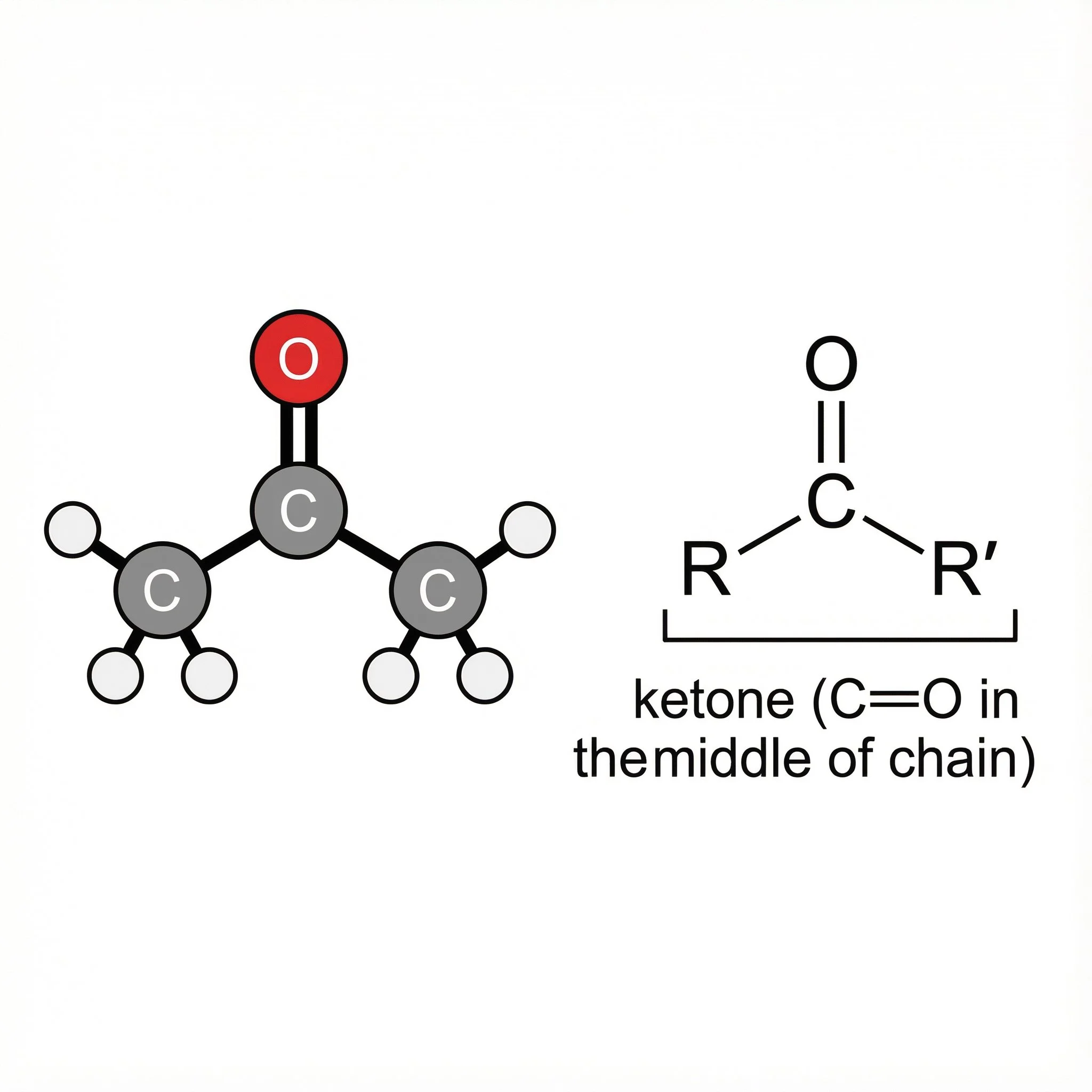
Ketones (>C=O) – carbonyls in the middle of a chain, more settled but still ready to react under the right conditions.
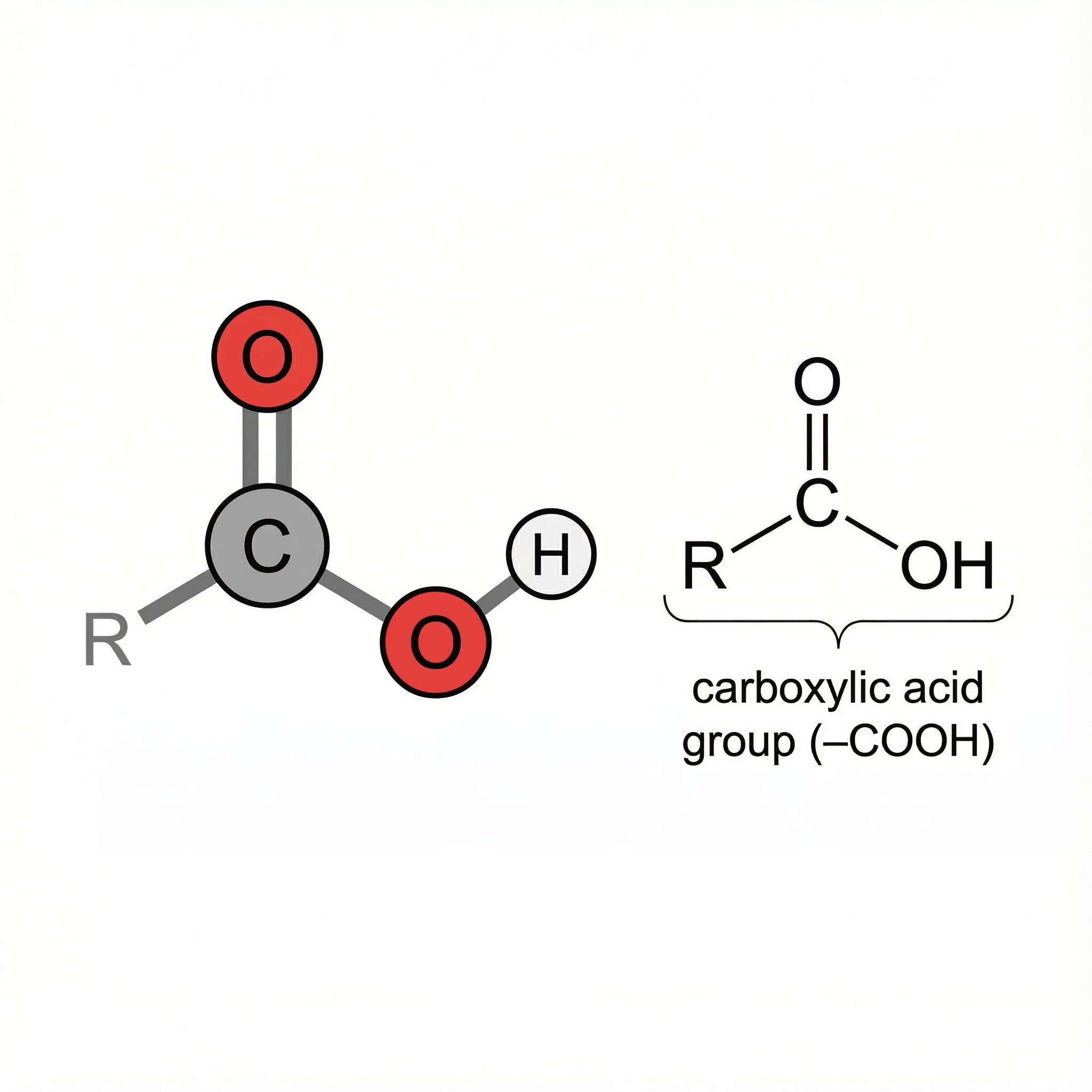
Carboxylic acids (–COOH) – carbonyl plus O–H on the same carbon, genuinely acidic and often sour, from vinegar to fruit acids.
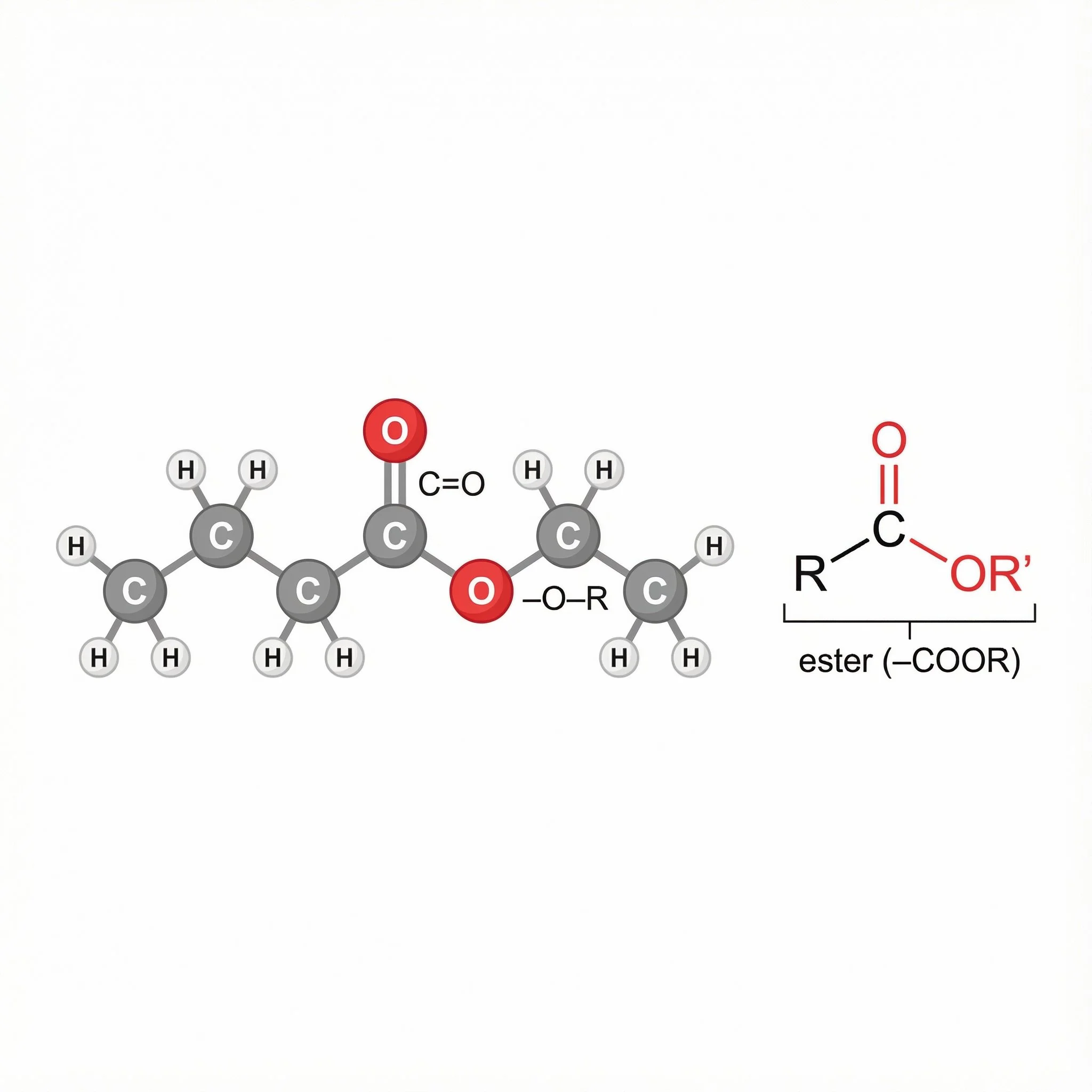
Esters (–COOR) – carbonyls linked to an –OR group, the “fruity perfume” family in many essential oils and plant aromas.
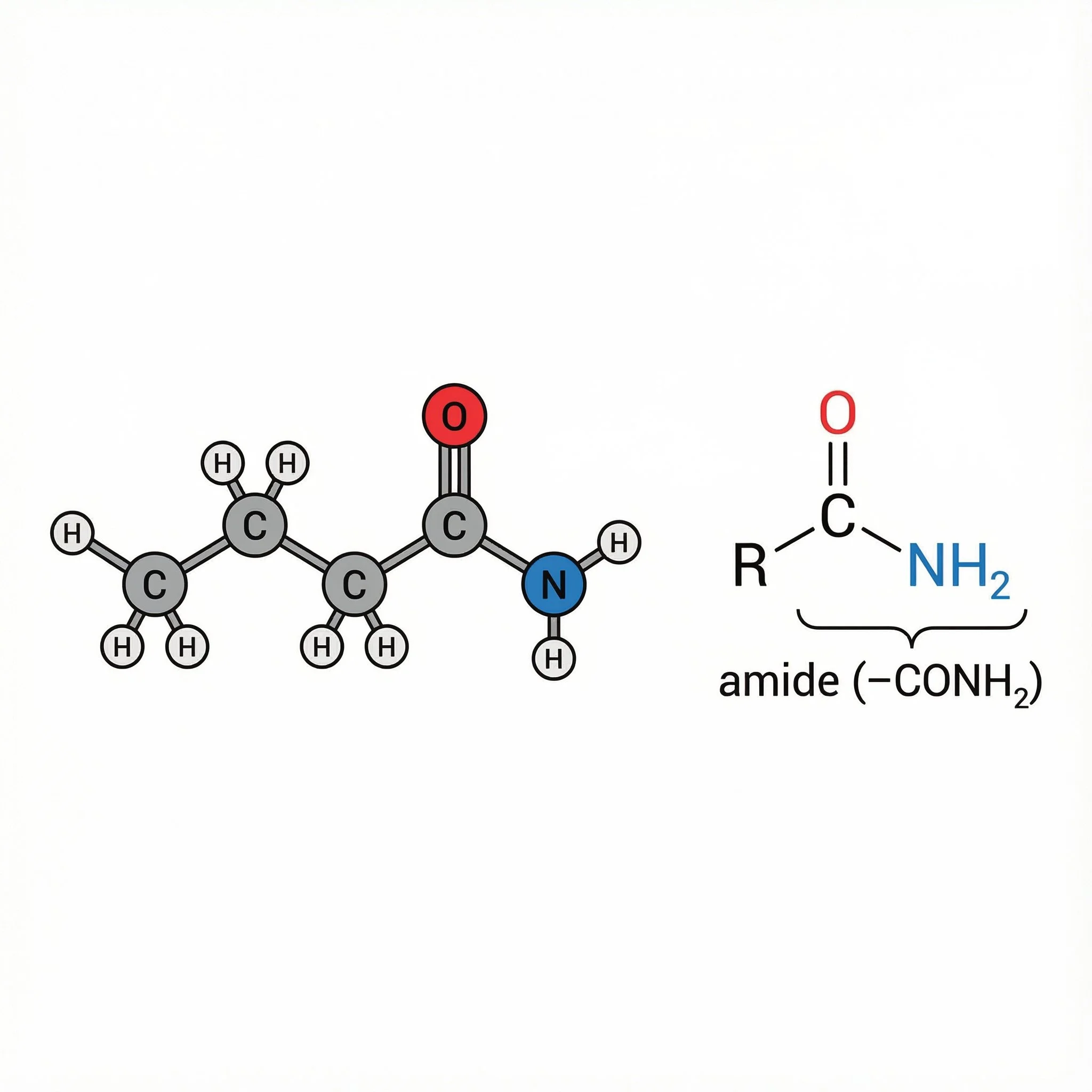
Amides (–CONH–) – carbonyls bonded to nitrogen, generally calmer and more stable, important in peptides, proteins and some plant alkaloids.
Each of these keeps the same C=O core, but what’s attached to it changes the polarity, reactivity and the kind of roles it plays in plant chemistry and herbal medicine.
Nitrogen-Based Groups
Nitrogen-based functional groups change how a molecule behaves: they can add basicity, charge and new bonding options, which affects how compounds interact with receptors, enzymes and solvents.
In herbal chemistry, nitrogen shows up most famously in alkaloids and amine-containing compounds, which are often more pharmacologically “punchy” than neutral plant constituents.
On this page we’re focusing on three key nitrogen groups:
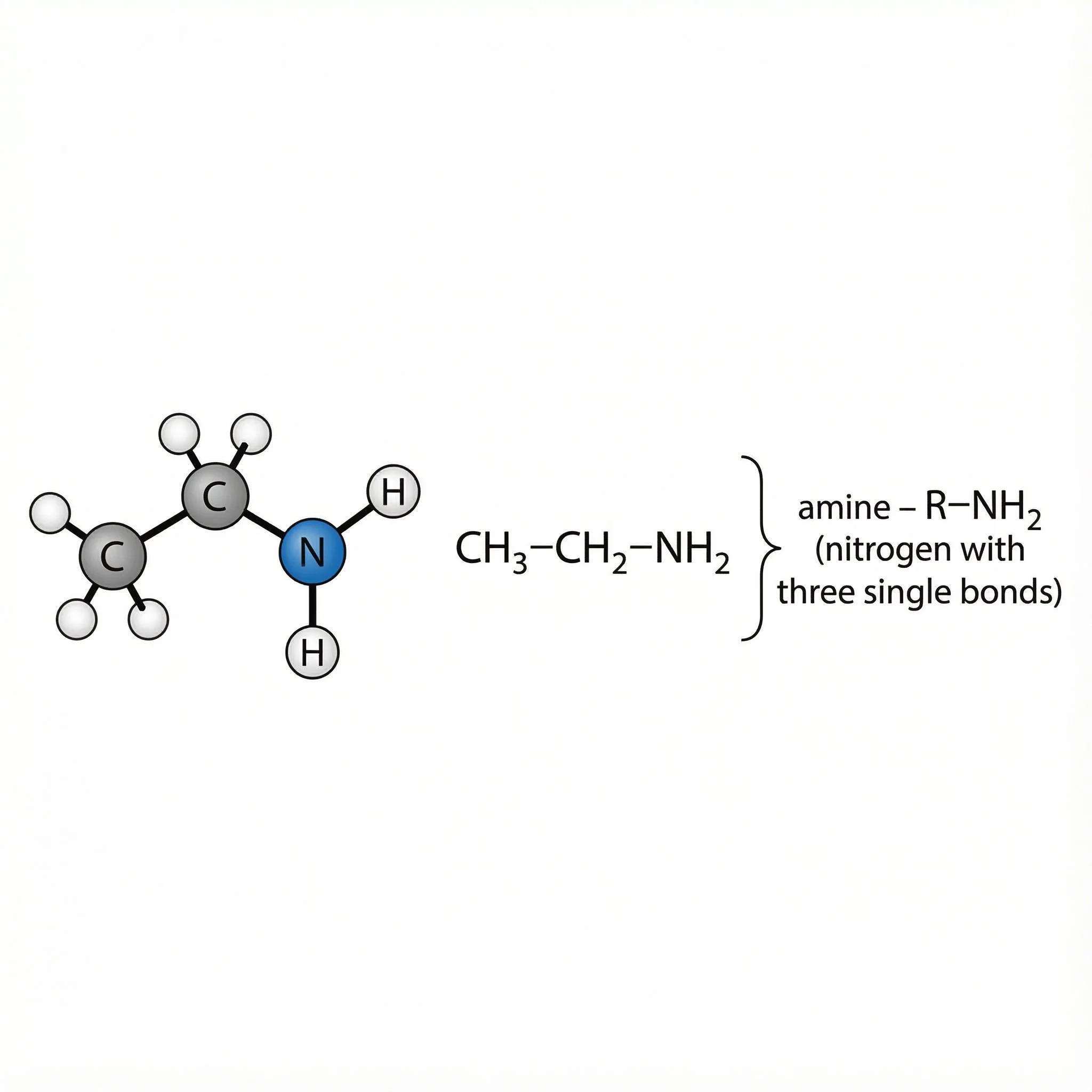
Amines (–NH₂, –NHR, –NR₂) – nitrogen analogues of alcohols, often basic and able to form –NH₃⁺, important in many bioactive plant compounds.
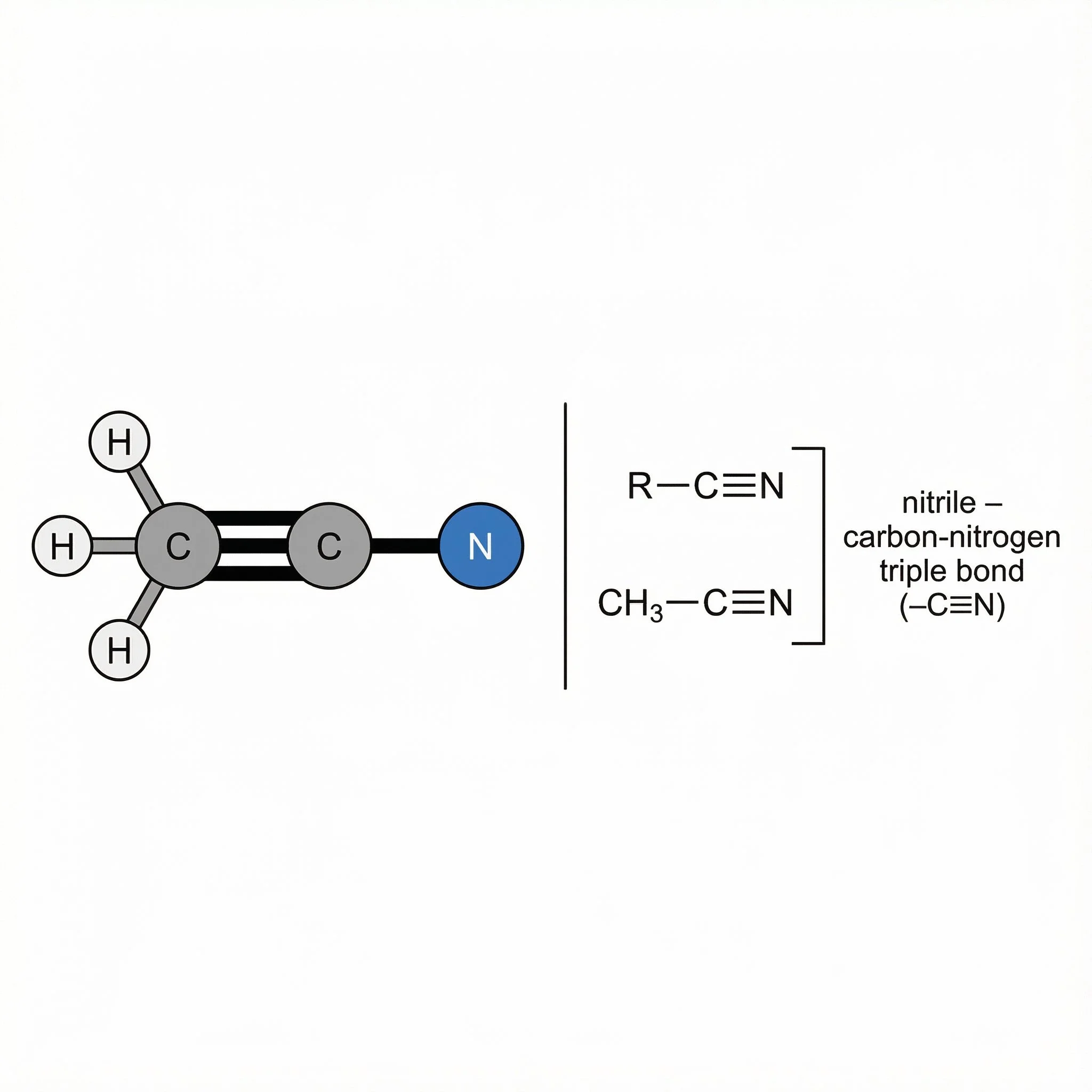
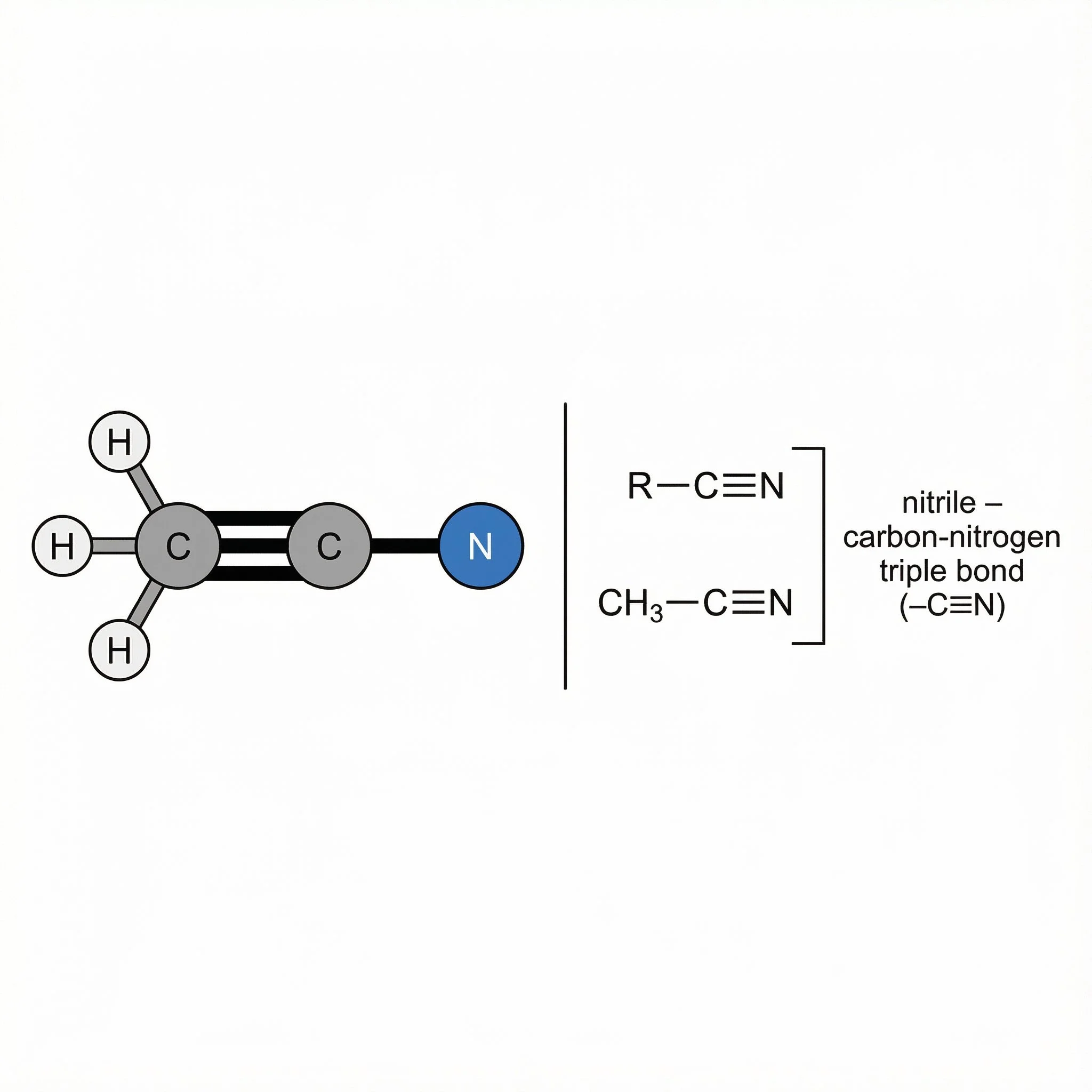
Nitriles (–C≡N) – carbon–nitrogen triple bond, relatively polar but not strongly basic; useful to recognise in natural and synthetic molecules.
Isocyanates (–N=C=O) – highly reactive N=C=O group, more of a “handle” in synthetic chemistry but worth being able to spot in reaction schemes and mechanisms.
Sulphur-Based Groups
Sulfur-based groups are less common than oxygen or nitrogen in organic molecules, but when they do appear they’re very noticeable. In plant chemistry, sulphur is responsible for many of the pungent, “sulphurous” aromas in garlic, onions and brassica vegetables, and underpins a lot of their biological activity.
Here we’re focusing on just one sulphur functional group:
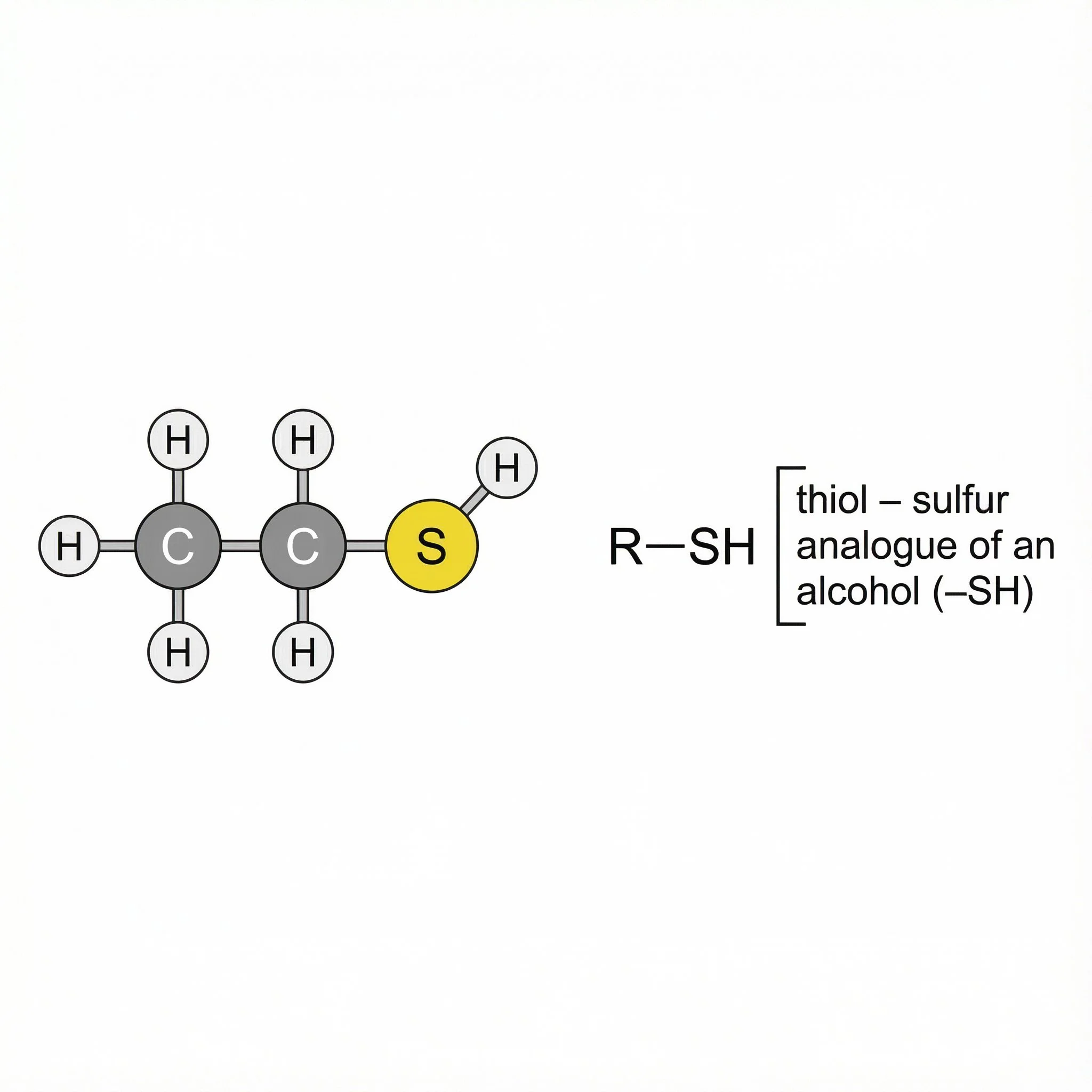
Thiols (–SH) – the sulphur analogue of an alcohol, with an –SH group attached to carbon. Thiols are often strongly odorous and good nucleophiles, and they’re a useful starting point for understanding the more complex organosulfur compounds found in plants such as Allium species (garlic, onions).
Aromatic
Aromatic rings (arenes) change how a molecule behaves: their delocalised electron system gives extra stability, characteristic substitution reactions and a very recognisable “aryl” backbone that other functional groups can be built onto.
In herbal chemistry, benzene-based rings appear in many major constituent families, especially phenylpropanoids, flavonoids, tannins and lignans, and often act as the core scaffold that carries hydroxyl, methoxy, sugar and other groups.
On this page we’re focusing on one key aromatic group:
Arenes (aryl rings, e.g. benzene) – six-membered carbon rings with conjugated double bonds and delocalised electrons, typically drawn as a hexagon with a circle or alternating double bonds, forming the aromatic core of many plant-derived compounds.