Simple Oxygen Heteroatoms
Alcohols and ethers are simple oxygen groups made from the same ingredients, just rearranged: alcohols are R–O–H, with that O–H group making them polar, reactive and right at home in teas and tinctures, while ethers are R–O–R, with oxygen sitting quietly in the middle as C–O–C, joining two carbons without causing as much fuss. In herbs, O–H is behind many water/ethanol-soluble, strongly flavoured, antioxidant-rich constituents, whereas ether and aromatic ether patterns turn up more in the oily, volatile and glycosidic side of plant chemistry.
Welcome to The O–H Arms and Club Ether, same elements, very different herbal personalities!
Alcohol - “The O–H Arms”
Welcome to “The O–H Arms”, what will it be?
The O–H Arms: Home of the alcohols.
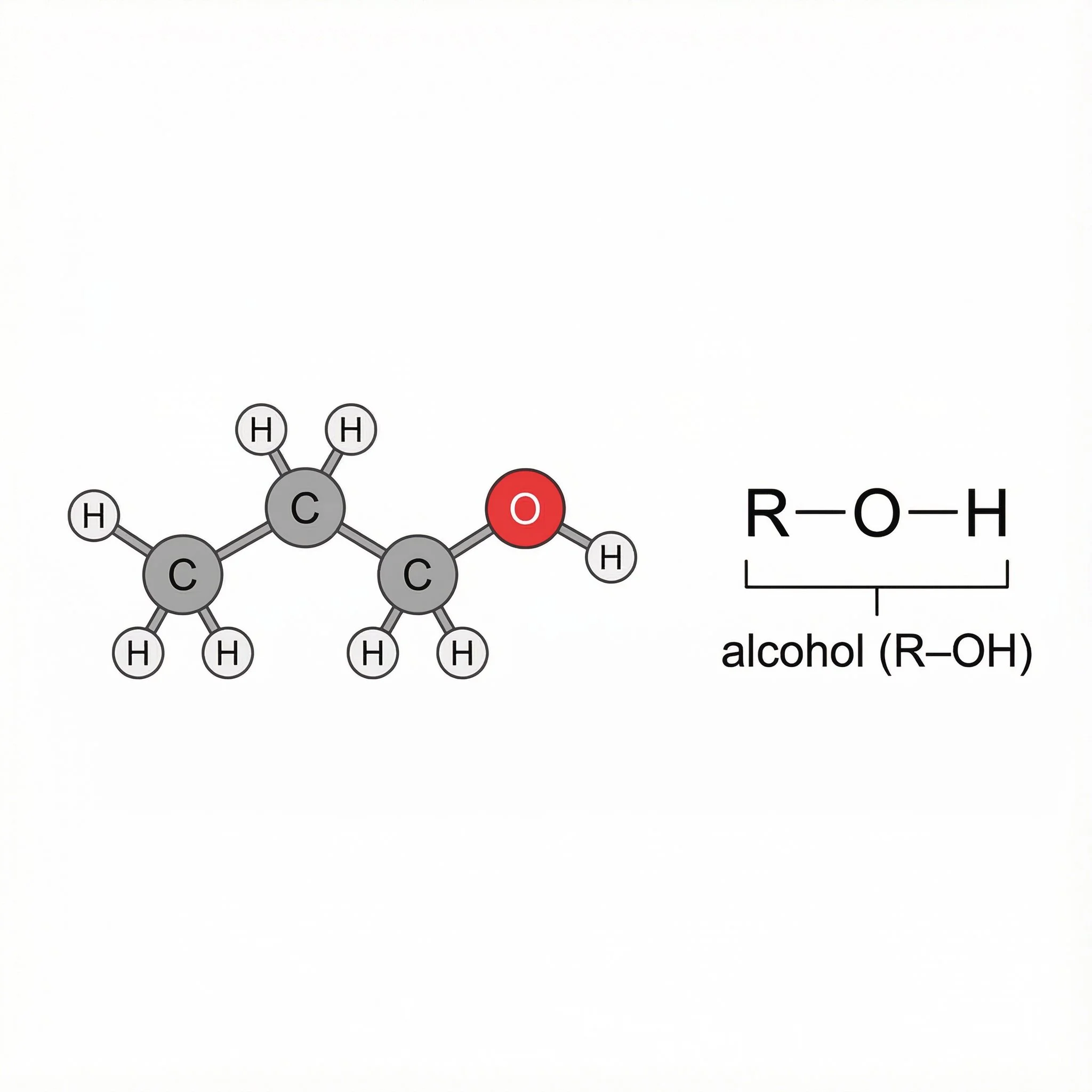
Every character in this pub is an ethanol molecule, a ball-and-stick body with a glowing O–H bulb stuck its head. That O–H is the functional group that makes an alcohol an alcohol.
Use the details in the scene as memory hooks:
The pub name “The O–H Arms” and neon O–H sign:
If you can see O–H on a carbon chain, you’re in alcohol territory.
Pattern: R–O–H.The blackboard rule:
“Add O–H to a carbon chain - Alcohol. O–H on the end = drama.”
That’s your one-line exam answer.The beer list: Ethan-Ale, Propan-Ale Bitter, Butan-Ale Lager, Iso-Prop Ale. Reminder that changing the carbon chain gives different alcohols, but the O–H group is always the key feature.
The grumpy regulars, spilled pints and near-fight: a visual nudge that once O–H turns up, the molecule is more interactive and reactive than a plain hydrocarbon, higher boiling point, better mixing with water, more chemistry going on.
An O–H group hanging off a carbon chain = alcohol (R–O–H).
Why O–H makes things more dramatic and why herbalists should care
In chemistry terms, adding an O–H group to a carbon chain changes everything:
Oxygen is electronegative, so the O–H bond is polar (O slightly −, H slightly +).
Alcohols can hydrogen bond with each other and with water
higher boiling points than the matching alkane
better mixing with water, especially small ones like ethanol.
The O–H group can take part in reactions:
it can be deprotonated (behaves a bit like a weak acid)
the carbon attached to O–H can be oxidised (primary alcohol - aldehyde - carboxylic acid).
In herbal terms, all that O–H drama means:
Molecules with O–H groups are often extractable in teas, decoctions, tinctures and glycerites.
Phenolic O–H groups contribute to antioxidant / radical-scavenging behaviour.
Lots of O–H usually means stronger flavour and mouthfeel – bitterness, sharpness or astringency.
O–H affects how a constituent interacts with proteins, enzymes and cell membranes, shaping its “chemical character” in the body.
So when you see that glowing O–H in a structure, think:
Exam brain: “Alcohol – R–O–H, polar, reactive.”
Herbal brain: “Teas/tinctures, noticeable taste, chemically ‘present’ in the body.”
That’s why The O–H Arms looks rowdier than Club Ether – the O–H crowd really are more dramatic.
Ether - “Club Ether”
Welcome to “Club Ether”
Club Ether – home of the ethers.
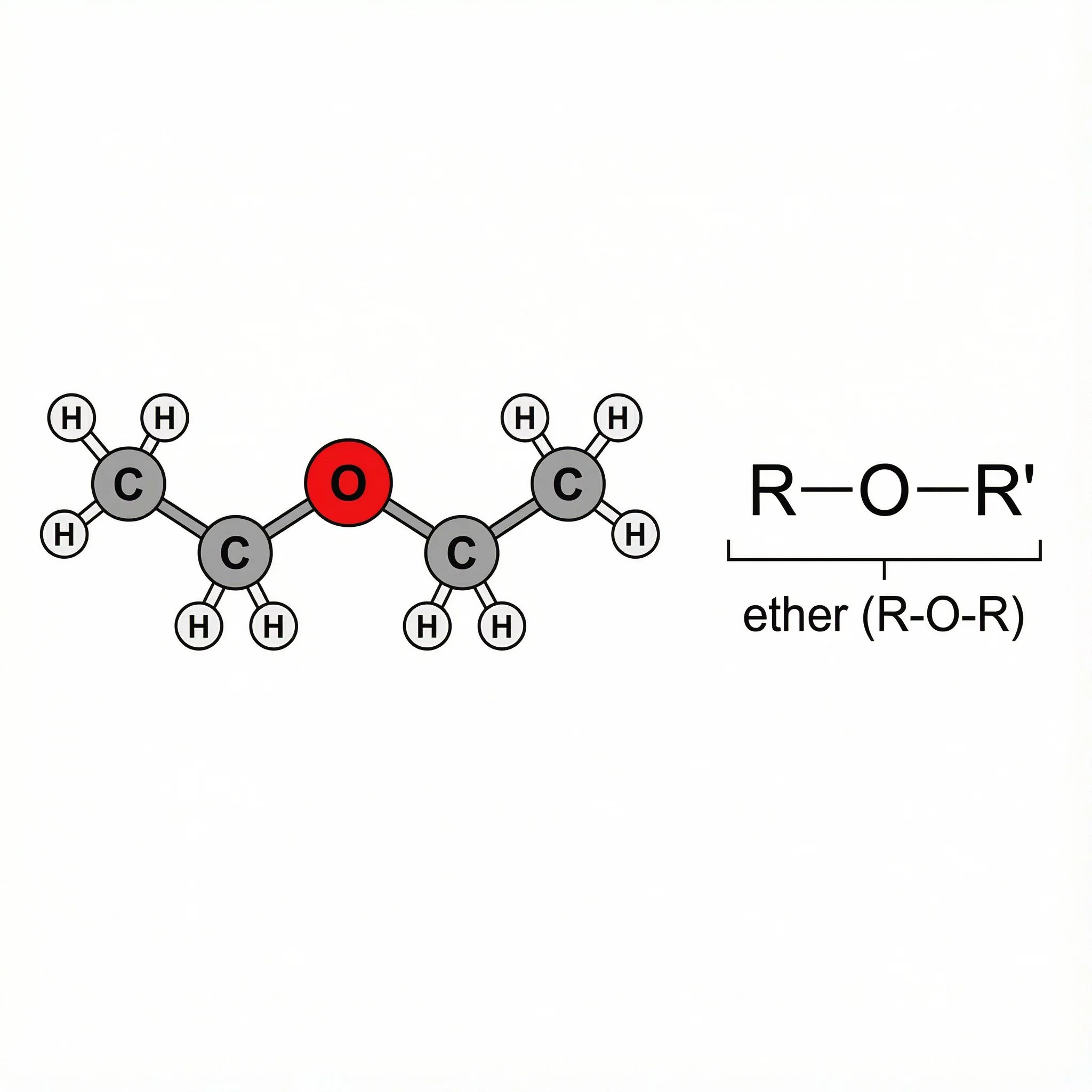
Everyone queuing on the red carpet and performing on stage is an ether molecule, ball-and-stick bodies built around a C–O–C pattern, with oxygen in the middle joining two carbon groups. Same elements as the alcohol crowd, very different layout.
Use the scene as your set of memory hooks:
The neon “CLUB ETHER” sign and the big marquee reading
“ETHER C–O–C IN THE MIDDLE. Same atoms as alcohol. Oxygen in the middle, less drama.”
If you can see C–O–C with no O–H, you’re in ether territory.
Pattern: R–O–R.The glamorous headliner on stage with a ball-and-stick dimethyl ether body:
one red O in the centre, a grey carbon group each side, hydrogens hanging off, literally C–O–C.The calm queue, red carpet and very serious bouncer: your reminder that, compared with the bar fight at The O–H Arms, ethers are smoother and less reactive. Same crowd, better organised.
An oxygen single-bonded to two carbons (C–O–C) with no O–H = ether (R–O–R).
Why C–O–C is “less drama” and why herbalists should care
The Chemistry bit:
Switching from O–H to C–O–C changes the behaviour:
Ethers follow the pattern R–O–R – oxygen is bonded to two carbons, with no hydrogen on O.
They’re still slightly polar (oxygen can accept hydrogen bonds), but they can’t donate them like alcohols do.
So they usually have lower boiling points than the equivalent alcohol.
They’re often less soluble in water and more volatile.
With no easily lost proton and no obvious oxidation target at O–H, ethers are generally less reactive under normal organic conditions, they tend to sit there and act as solvents or structural links rather than the star of the reaction.
The Herbal bit:
That calmer C–O–C behaviour shows up in a couple of places in plant chemistry:
Some essential-oil constituents are aromatic ethers, basically fragrant benzene rings with a C–O–C side chain.
They’re oily, lipophilic and volatile, more at home in essential oils and high-alcohol preparations than in a simple cup of tea.
Many plant molecules exist as glycosides, where a sugar is attached to an aglycone by a C–O–C (ether) bridge.
The sugar part makes the whole molecule more water-friendly, while the ether link is a relatively stable connection that can be cut by enzymes or gut microbes later.
That link helps control solubility, transport and when/where the active part is released.
So when you spot C–O–C with no O–H in a structure, think:
Exam brain: “Ether – R–O–R, oxygen in the middle, less reactive than an alcohol.”
Herbal brain: “More on the oily/volatile or linker side of life, think essential-oil style behaviour or sugar, aglycone bridges, not big dramatic water-loving polyphenol energy.”